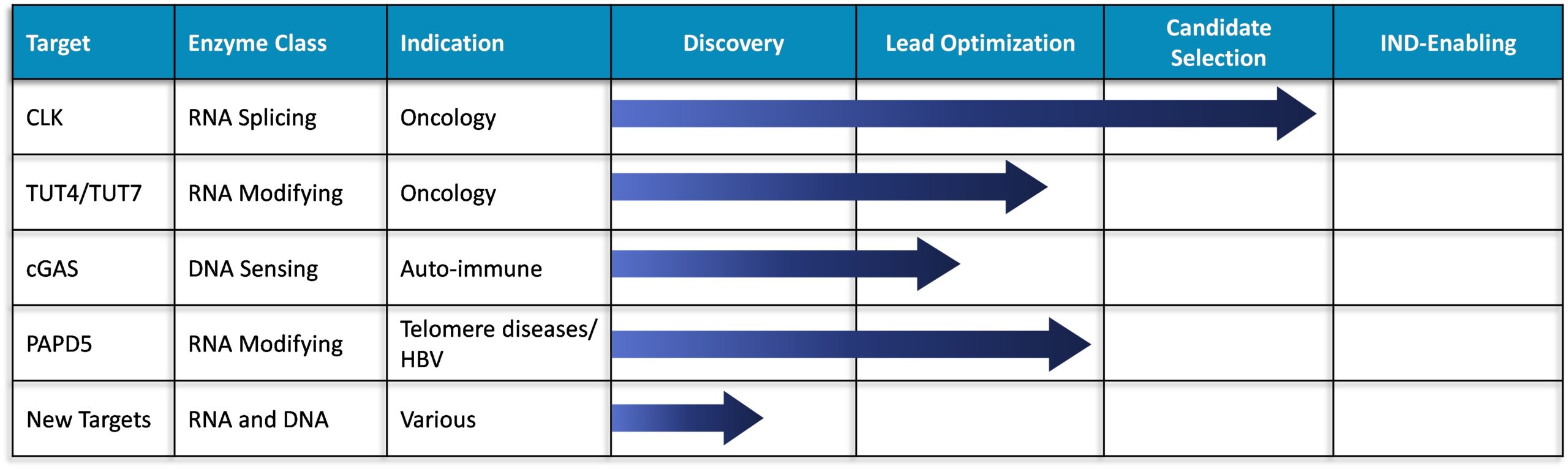
Exciting Pipeline of Novel Programs
Our toolbox was successfully applied to a diverse set of nucleic acid enzymes to rapidly generate and advance a series of exciting target programs.
Redona has developed a unique toolbox to rapidly generate robust and compelling small-molecule inhibitor programs targeting these novel nucleic acid processing and sensing enzymes. This toolbox includes an expanding set of rigorously validated chemical and biological reagents, a proprietary and extensive suite of biochemical and cellular assays, a highly curated and defined chemical motif library that efficiently interrogates nucleic acid – protein interactions, and strong bioinformatic capabilities to integrate and understand the complex biology impacted by these nucleic acid regulators.

Our toolbox was successfully applied to a diverse set of nucleic acid enzymes to rapidly generate and advance a series of exciting target programs.


Over 95% of RNA is alternatively spliced to create a diversity of protein products from a given gene. Aberrant RNA splicing occurs in a number of cancers and is believed to be a driver of oncogenesis. There is growing understanding of specific proteins and other factors involved in aberrant splicing and their role in diseases.
CLK (CDC-like kinase) is a family of four enzymes (CLK 1 – 4) that acts on proteins in the spliceosome complex. CLK over-expression has been functionally tied to aberrant RNA splicing in a variety of cancers including solid tumors (lung, breast, colorectal, pancreatic) and heme malignancies (AML, CML, MDS). CLK inhibitors have demonstrated the ability to inhibit cancer cell growth and induce apoptosis through splicing alternations in genes involved in growth and cell survival.
Redona has several potent and selective pan-CLK inhibitor leads that have demonstrated compelling activity in cellular and animal models of cancer. The leads are orally bioavailable and currently undergoing additional profiling for nomination of a development candidate in the near future.
RNA homeostasis is a highly regulated process governed by a multitude of cellular pathways. The rates of RNA synthesis and degradation are among the most important factors for gene regulation. Poly-uridylation of the 3’-end of mRNA is well established as one of the key mechanisms to mark RNA for degradation by various exonucleases.
RNA uridylation is performed by various terminal uridyl transferase (TUT) enzymes. TUT4 and TUT7 function redundantly and are found to be upregulated in certain cancers. Redona has developed a series of potent and selective dual inhibitors of both TUT4 and TUT7 (TUT4/7). Genetic ablation as well as pharmacological inhibition of TUT4/7 have demonstrated compelling efficacy in both cell and animal cancer models. In addition, a strong association between TUT4/7 sensitivity and specific genetic deletions found in certain cancers has been identified that will aid in developing a robust clinical strategy for these compounds.
cGAS (cyclic GMP-AMP synthase) is a sensor of cytosolic DNA generated by infections, tissue damage, cancer, and auto-immune diseases. Activation of the cGAS signaling pathway leads to the expression of pro-inflammatory genes including type 1 IFN and NF-kB. The broad activation of these pathways by cGAS provides potential therapeutic opportunities for intervention in a wide range of indications including autoimmune diseases and cancer.
Redona’s toolbox has enabled the rapid development of series of inhibitors that are potent and selective against cGAS, that cross-react between human and mouse targets, and possess excellent cell penetration and oral PK properties.
PAPD5 and PAPD7 are members of a family of poly(A)polymerases that function by appending multiple adenyl groups onto the ends of RNA. PAPD5/7 are cellular host factors required for HBV RNA stabilization via poly-adenylation. As such, PAPD5/7 inhibitors are considered an important component of HBV combination therapy needed to produce a functional cure. In addition, PAPD5 poly-adenylates and destabilizes TERC (telomerase RNA component), an important factor required for telomere maintenance. Inhibition of PAPD5 in this context has application in rare genetic diseases such as dyskeratosis congenita that may lead to bone marrow failure, liver cirrhosis and pulmonary fibrosis.
Redona has developed an advanced series of highly potent and selective PAPD5/7 inhibitors that contain a unique chemical scaffold relative to the competitor molecules. These lead compounds have excellent oral bioavailability and have demonstrated activity in animal models of HBV infection.




1

Our goal is to become the leader in the therapeutic regulation of RNA.
We welcome the opportunity to: